Aldehydes
Before we started class we had to recognize what R stands for.
R= Radical (which is any carbon molecule)
We recognized that in order for a molecule to be an Aldehyde it must have a Carbonyl group.
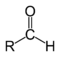 Also recognized as -CHO
Also recognized as -CHOWhen naming aldehydes:
- Identify the longest continuous chain of carbons with the carbonyl carbon as part of the chain.
- Number the carbon chain so that the carbonyl carbon is always number one.
- Locate and identify alphebetically the branched groups by prefixing the carbon number it is attached to. If more than one of the same type of branched group is involved use the prefixes di for 2, tri for three, etc.
- After identifying the name, number and location of each branched group, use the alkane name that represents the number of carbons in the continuous chain.
- Change the "e" ending and replace it with -al
It is like naming an alkane but only with a different suffix.
Example #1
 propanal  2 methyl-propanal Functional Group:The group of atoms responsible for the characteristic reactions of a compound. It is basically what makes that molecule special.
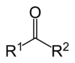
Original Names, the most used name in the lab. Acetaldehyde- Ethanal Formaldehyde- Methanal etc. Ketone There are two forms of naming a ketone: formally and commonly. They are as follows: Naming ketones formally:
2. Name it as an alkane 3. Drop the suffix "e" and add "one" 4. Name it alphabetical order 5. The Carbon attached to the Oxygen should be relatively in the middle of the structure. This website can help you : http://www.cartage.org.lb/en/ In naming ketones commonly,
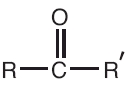 Ketones structural formula (Carbonyl group), notice there is no Hydrogen. Ketones structural formula (Carbonyl group), notice there is no Hydrogen.Example #2  this is called 3,Methyl- 2, Butanone this is called 3,Methyl- 2, Butanone this is called diethyl ketone. this is called diethyl ketone. this is called :1) 2-pentanone or 2) methyl 1-propyl ketone |
Stefano Pinzon

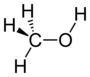
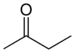



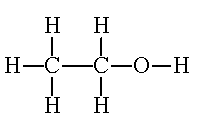 As it says in the picture it is ethanol.
As it says in the picture it is ethanol.









