In today's class we began learning the Molecules involving Carbon +Hydrogen+ Oxygen. In others words, Alcohols.
You must understand that all alcohols have a hydroxyl group. A hydroxyl group is an Oxygen attached by a covalent bond to a single Hydrogen.
Alcohols use the same formats as alkanes. To name alcohols,
- Determine the parent chain. The parent chain must be the longest that includes the carbon holding the OH group.
- Number according to the end closest to the -OH group regardless of where alkyl substituents are.
- The format is as follows: (location of branch)-(branch name)-(location of OH group)-(parent chain)
- Change the parent chain -e ending and replace it with an -ol.
Examples:
 In this case it is called Methanol, due to is single Methyl and single OH.
In this case it is called Methanol, due to is single Methyl and single OH.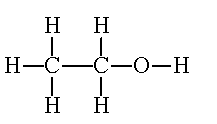 As it says in the picture it is ethanol.
As it says in the picture it is ethanol.CH3-CH2-CH2-OH In this case it is called 1-Propanol.
OH | CH3-CH-CH3 In this case it is called 2-Propanol.
This is a harder example to identify: H | H H H-C-H H | | | | H-C-C---C---C-H | | | | H O H H | H Parent chain: butane -OH group location: 2 Substituents locations: 3-methyl Alkane name: 3-methylbutane Alcohol name: 3-methyl-2-butanol!!!!!!!!!!!!!Just to make clear as i had done it wrong as well at the beginning is to add ol after the alkane has been name. This next example should clarify any problems, and if you are still confused please ask me!!!!!!!!!!!!!!!!!!!!
Alchohols containing more than one hydroxyl group are also called polyalcohols. Polyalcohols are named similarly to alcohols, with the exception of the prefix di-, tri-, etc before the -ol ending.
Example:
Example:
H H | | H-C-C-H | | OH OH This is called 1,2-ethanediol
H H H | | | H-C-C-C-H | | | OH OH OH This is called 1,2,3-propanetriol
Kinds of Alcohols
There are three types of Alcohols:
1) Primary: an alcohol which has the hydroxyl radical connected to a primary carbon.
2) Secondary: an alcohol with its hydroxyl radical connected to a primary carbon which is also connected to another carbon.3) Tertiary: An alcohol in which the hydroxyl group is attached to a carbon that is joined to three carbons. 
Ethers
Ethers was the easiest part of the class. There will always be an Oxygen in the middle, so to speak. You identify
the components and add ether without adding coordinates (no numbers).
For example: CH3-O-CH2-CH3 In this case it is called ethyl-methyl-ether. simple.
CH3-CH2-O-CH2-CH3 In this case it is called diethyl ether.
Extra Information: when you add a hydroxyl group to Benzene it is called a phenolStefano Pinzon
No hay comentarios:
Publicar un comentario